Atomic Physics
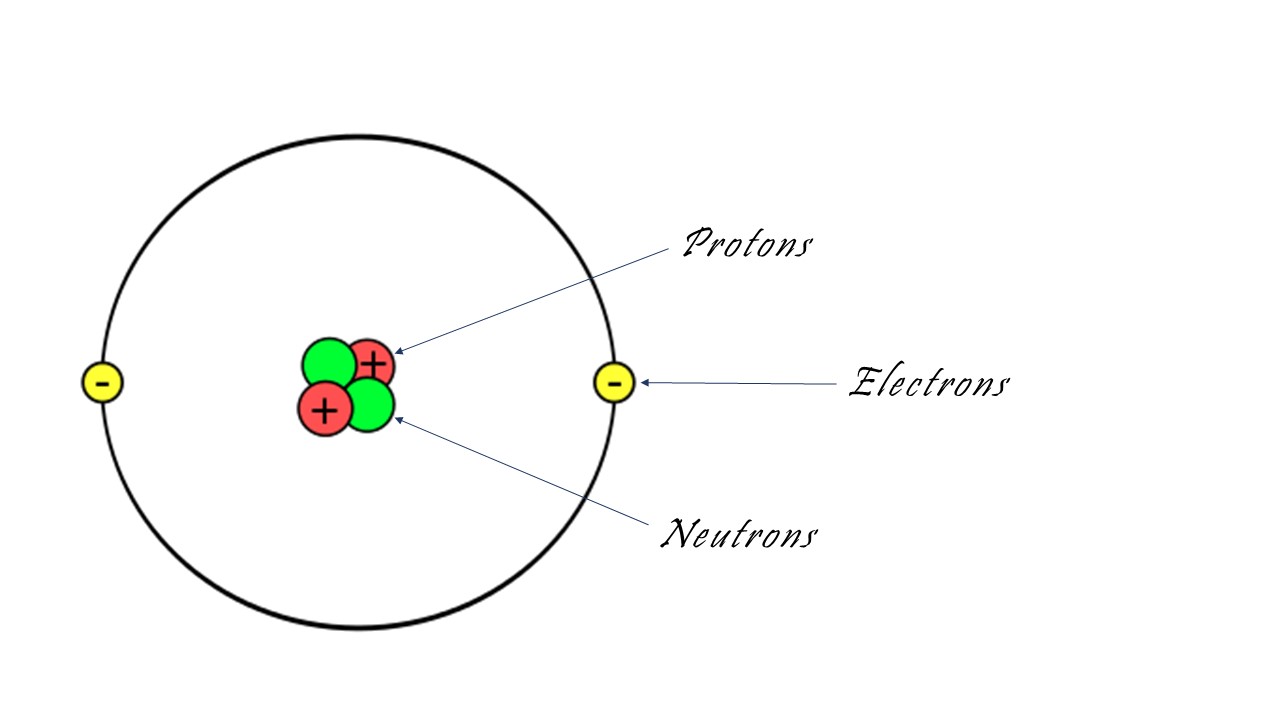
Atoms are the smallest units of ordinary matter that form a chemical element. All solids, liquids and gases consist of atoms. An atom is about 100 picometers in diameter.
Atoms are composed of a nucleus made of one or more protons and a number of neutrons, and one or more electrons bound to the nucleus. The protons are positively charged particles and the electrons are negatively charged. About 99% of an atom's weight is in the nucleus. Atoms are indivisible. Atoms of each element have different properties and combine in predictable ratios.
 This model is also described as the billiard balls model. In 1803,
John Dalton conducted experiments with gases and used the results to propose the modern theory of the atom based on the following assumptions.
This model is also described as the billiard balls model. In 1803,
John Dalton conducted experiments with gases and used the results to propose the modern theory of the atom based on the following assumptions.
 This model is also described as the plum pudding model. The positive charges fills the atom while the electrons were embedded throughout the atom.
Thomson discovered the electron and since the electron was negative, but atoms neutral, there had to be positive charge inside atoms. Thomson used a beam of
cathode rays in a CRT with both an electric field and a magnetic field perpendicular acting on the beam. With only the electric field on, the beam was
deflected toward the positive plate. With only the magnetic field on, the cathode rays were deflected into a curved path. When both fields were on, and the field
strengths equal, the cathode rays were not deflected.
This model is also described as the plum pudding model. The positive charges fills the atom while the electrons were embedded throughout the atom.
Thomson discovered the electron and since the electron was negative, but atoms neutral, there had to be positive charge inside atoms. Thomson used a beam of
cathode rays in a CRT with both an electric field and a magnetic field perpendicular acting on the beam. With only the electric field on, the beam was
deflected toward the positive plate. With only the magnetic field on, the cathode rays were deflected into a curved path. When both fields were on, and the field
strengths equal, the cathode rays were not deflected.
 Around 1911 Rutherford, Marsden and Geiger performed experiments to test the Thomson model. They directed alpha particles from
radioactive sources onto thin gold foils. The Thomson model predicted that most of the alpha particles would go straight through, and only a few would be deflected
at small angles since the electrons in the atom have much less mass than alpha particles. Most of the particles went straight through undeflected,
some were deflected at angles of more than 10o and a few were deflected almost straight back. He concluded that most of the atom was empty space with most of the mass
and all of the positive charge concentrated in a very small region (the nucleus). Scattering angles indicated the size of the nucleus was about 1015 to 1014m in radius.
Around 1911 Rutherford, Marsden and Geiger performed experiments to test the Thomson model. They directed alpha particles from
radioactive sources onto thin gold foils. The Thomson model predicted that most of the alpha particles would go straight through, and only a few would be deflected
at small angles since the electrons in the atom have much less mass than alpha particles. Most of the particles went straight through undeflected,
some were deflected at angles of more than 10o and a few were deflected almost straight back. He concluded that most of the atom was empty space with most of the mass
and all of the positive charge concentrated in a very small region (the nucleus). Scattering angles indicated the size of the nucleus was about 1015 to 1014m in radius.
In Rutherford's model, electrons could 'orbit' the nucleus at any energy level. The closer the alpha particle is to the nucleus the greater its potential energy.
 The study of emission and absorption spectra led to the development of Bohr postulates. Incandescent solids, liquids and high pressure gases emit a continuous spectrum.
Low pressure gases emit a bright line spectrum when excited by heat or electricity. Each element has its own emission spectrum, which shows that the atoms of that element can
emit only certain photon energies.
The study of emission and absorption spectra led to the development of Bohr postulates. Incandescent solids, liquids and high pressure gases emit a continuous spectrum.
Low pressure gases emit a bright line spectrum when excited by heat or electricity. Each element has its own emission spectrum, which shows that the atoms of that element can
emit only certain photon energies.
A cool low pressure gas will absorb some wavelengths when white light is passed through it, resulting in a dark line i.e. absorption spectrum. The wavelengths that are absorbed match the emission spectrum. Each element has its own absorption spectrum which shows that the atoms absorb only certain photon energies.
Johann Jacob Balmer discovered an empirical equation that predicted the wavelengths of the 4 visible lines of hydrogen. Bohr used Balmer's equation to explain that the spectral lines corresponded to differences between quantized energy levels in the hydrogen atom, which led to the Bohr model of the atom. The Bohr model has three distinct principles:
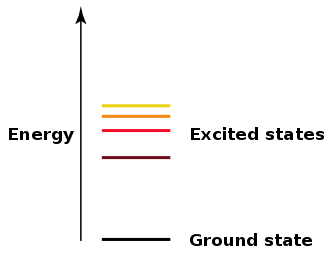
The most stable energy level of an atom is the Ground state. Atoms can absorb energy causing the electron to transition to a higher energy state. The atom is said to be excited. Excited electrons are not stable and they transition back to the ground state. The energy emitted by the electron as it transitions back to ground state is emitted as a photon called Florescence. When the electrons are at the infinite energy level, they have 0.00 energy.
In 1896, Henri Bequerel discovered that Uranium and other elements emitted invisible rays that can penetrate solid material. These materials are now called 'Radioactive'. Radioactivity is defined as the process by which atoms emit energy in the form of electromagnetic waves, charged particles or uncharged particles. The unit for radiation is counts per second, also known as a Becquerel (Bq). The Geiger counter is the device used to measure ionizing radiation.
Exposure to radiation is unavoidable because radioactive elements occur in nature. Some forms of carbon absorbed in our bodies are radioactive. Cosmic rays are high energy radiations from space and individuals who are flying at high altitudes get higher exposure. Radioactive elements like uranium and radium are found in soil and rocks.
Artificial sources of radioactivity include electricity, space probes and submarines. Medical equipment such as X-rays and gamma rays used in diagnosis and cancer treatment respectively.
The energy carried by ionizing radiations can break chemical bonds. These can cause mutations in DNA and these mutations have more impact on cells that are dividing rapidly. Mutation can cause cell damage, which may either result in cell death (apoptosis) or the damaged cells may be transmitted from parents to offspring causing genetic defects and/or malformations.
The strength of radiations depend on three factors: