Energy Flow in the Biosphere


Autotrophs are organisms that use energy from the sun and raw materials to make their own food. They are also known as producers.
Heterotrophs are organisms that are incapable of making their own food, so they must feed on other organisms to obtain energy.
Primary Consumers are consumers that rely directly on autotrophs for their source of energy.
Secondary Consumers are consumers that rely primarily on primary consumers for their source of energy.
Trophic level is the measure of how far an organism is from the original energy source. Such that plants (Autotrophs) are the first trophic level because their energy is from the sun. When an organism is consumed by another, energy is transferred. For instance Plant > Mouse > owl; Producer > primary consumer > secondary consumer respectively, and trophic levels T1 > T2 > T3 respectively.
Food Chain is a linear expression that indicates how energy is transferred from the producers and between consumers. The arrow points at the eater, and it separates the trophic levels.
A Food Web depicts a series of several food chains with multiple interactions this represents a more accurate energy pathways.
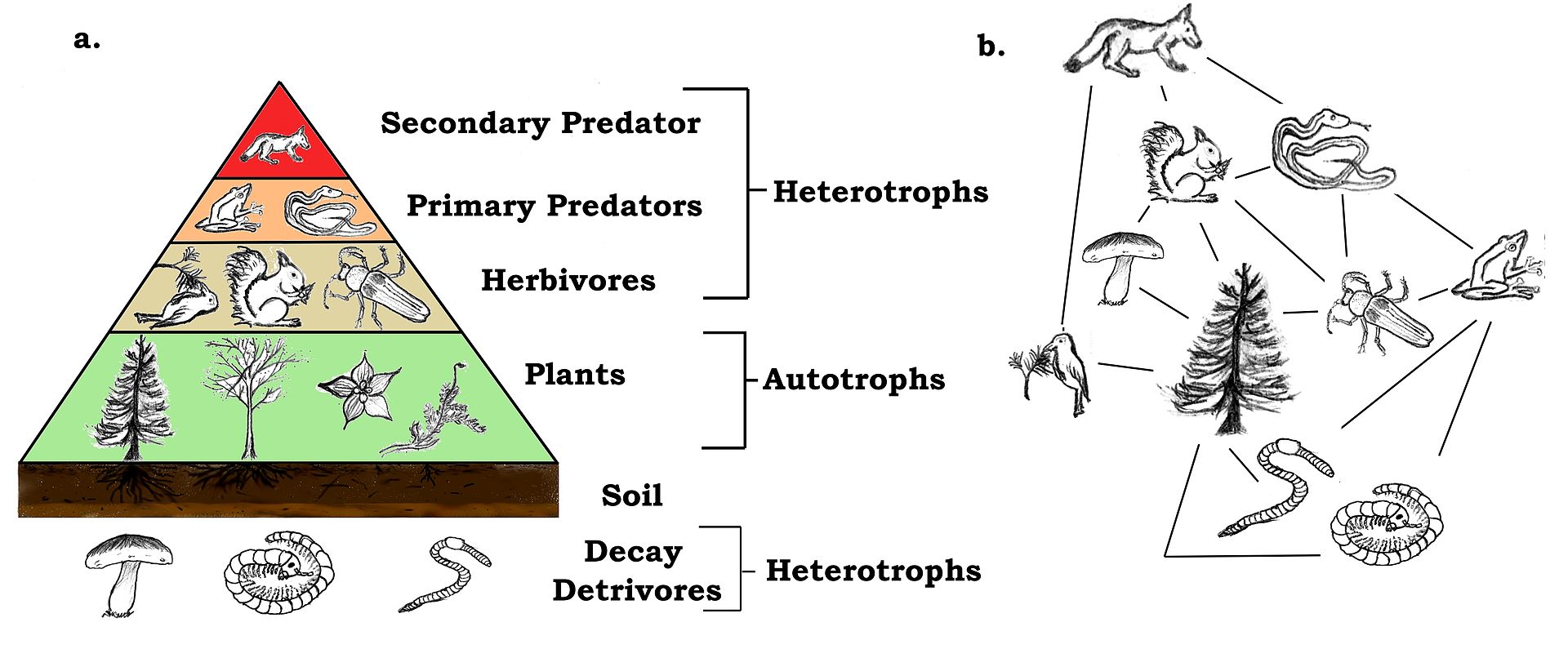
An example of a trophic web and a simplified food web. (Source: Wikipedia, CC BY-SA 3.0)
Herbivore is an organism that only eats plants. Includes Rabbit, Deer.
Carnivore is an organism that only eats animals. Includes Wolves, Tigers.
Omnivore is an organism that can eat both plants and animals. Includes Bears, Humans.
Scavenger is an organism that feeds on dead organisms or the wastes of organisms. Includes Vultures, Seagulls.
Decomposer is an organism that breaks down organic wastes and the remains of dead organisms into simpler compounds such as carbon dioxide, ammonia, Water.
Food chains always begin with autotrophs. Through photosynthesis, autotrophs obtain energy from the sun and store it as sugars, such as glucose, starch, sucrose etc.
The above formula shows how photosynthesis produces oxygen and provides energy to all other organisms. In addition, all organisms, including plants, undergo cellular respiration to use the energy stored in their food.
Chemoautotrophs are organisms that can produce energy from CO2 and water, but do not use energy from the sun so they obtain energy from chemicals such as ammonia, sulfur or hydrogen sulfide.
Energy flows only in one direction. For example, from plants to the deer or cattle, and not the other way round.
From one level to the next, only 10% of energy is transferred. This limits the number of trophic levels that can be present in a food chain.
First Law of Thermodynamics
Law of conservation of energy - Energy can be changed in form, but not created or destroyed. Energy input = Energy Output.
Second Law of Thermodynamics
Any energy change results in loss of energy as heat. i.e. Energy input = Useful energy + wasted energy.
Ecological pyramids are a visual representation of the interactions among the trophic levels in an ecosystem. The producer populations form the base of the pyramid; the primary consumers form the second level, followed by the secondary, tertiary, quaternary and higher levels of consumers.
Pyramid of Numbers: The total number of organisms.
Pyramid of Biomass: The total mass or dry weight of all living matter. Usually represented by the units g/m2.
Pyramid of Energy: The energy flowing through the levels. Usually represented by the units of energy i.e. J or KJ KJ/m2/year.
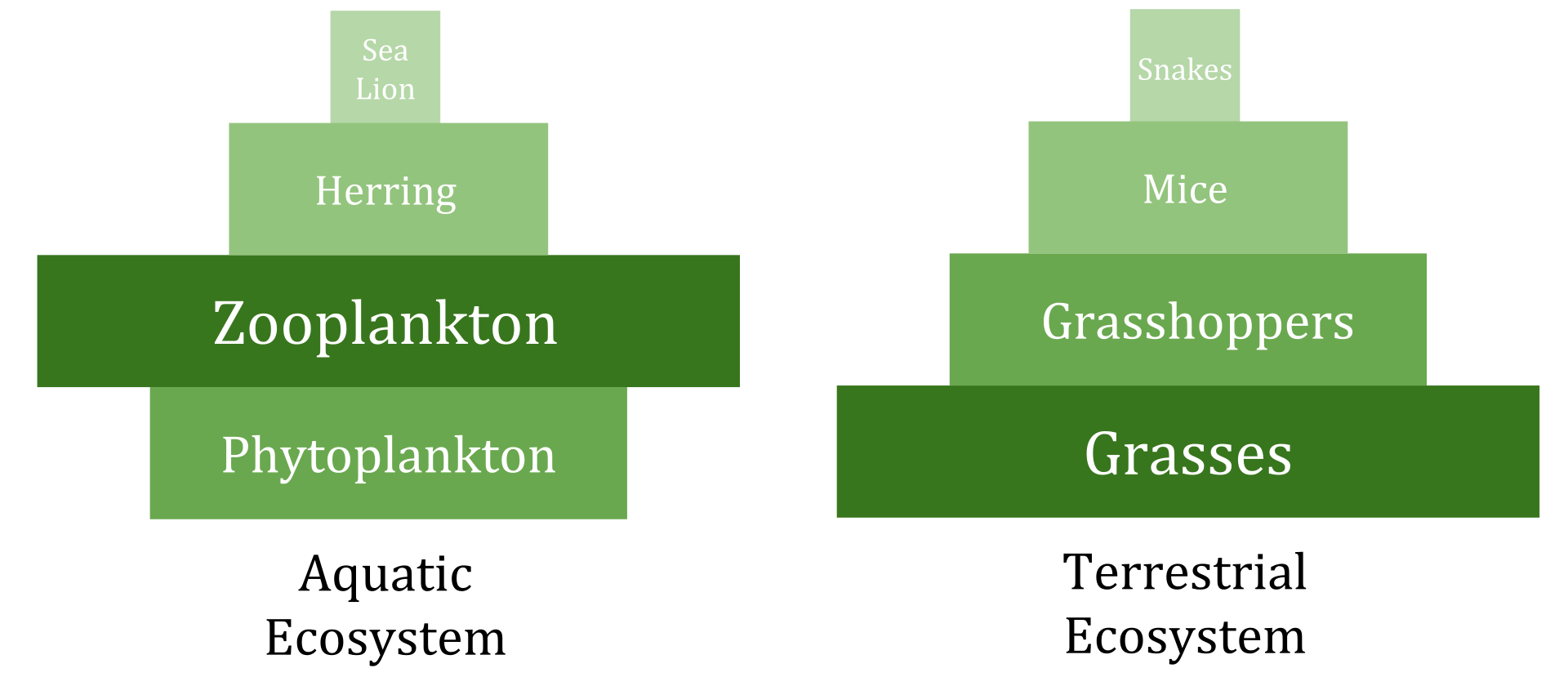
Examples of pyramid of numbers. (Source: Wikipedia, CC BY-SA 3.0)
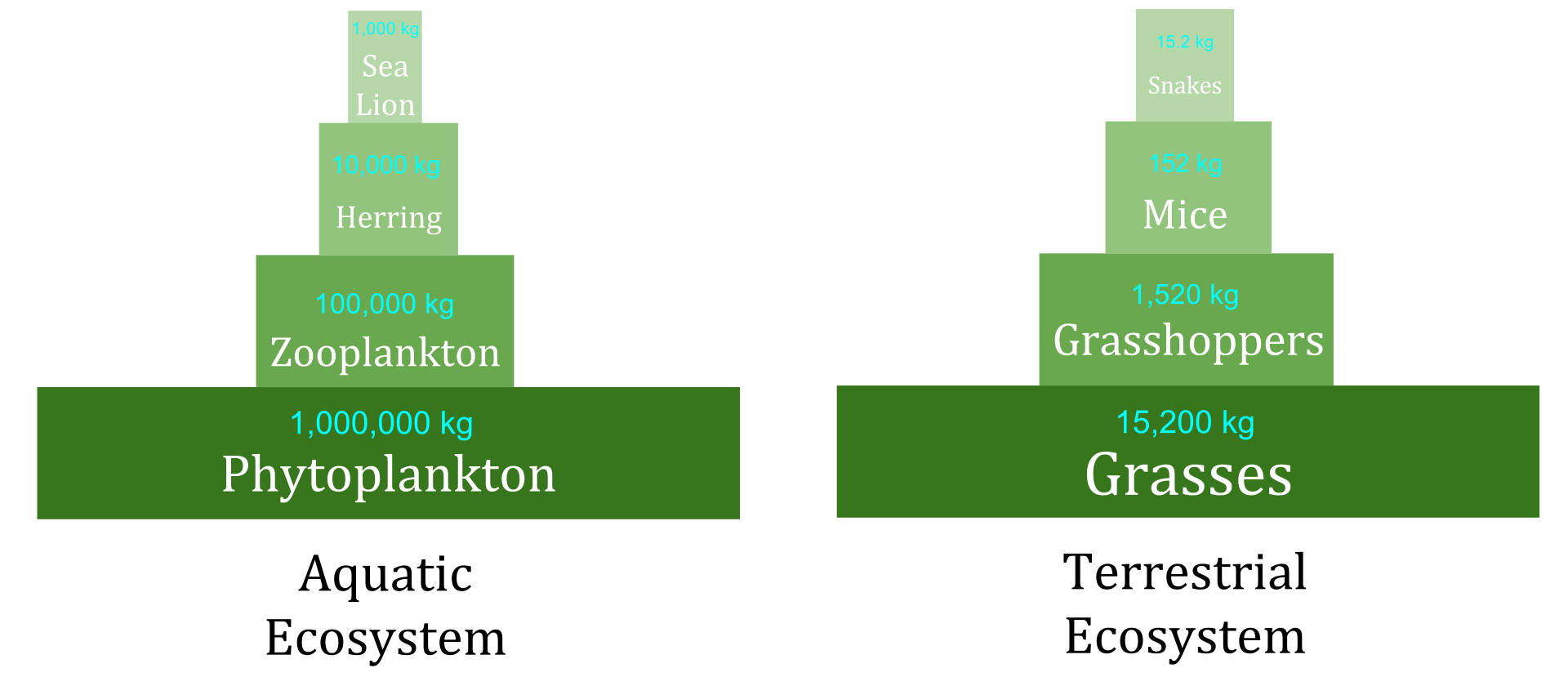
Examples of pyramid of biomass. (Source: Wikipedia, CC BY-SA 3.0)
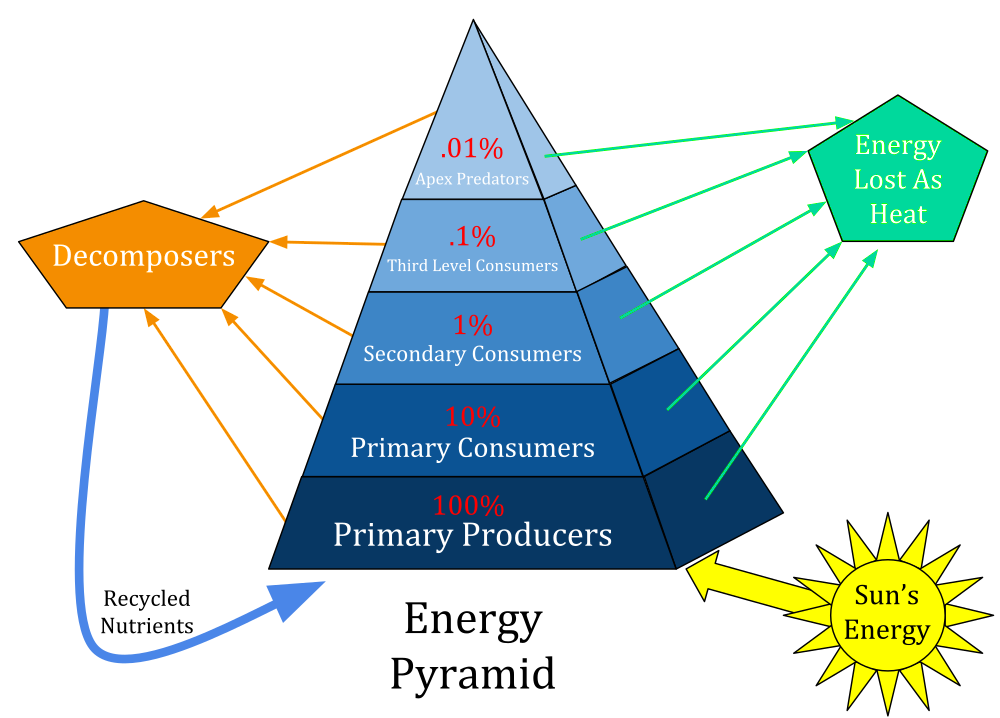
An example of a pyramid of energy. (Source: Wikipedia, CC BY-SA 3.0)
Download a pdf copy of Unit 3 - Energy Matter Biosphere for offline use.
C$0.99
Biochemical cycles are cycles for transferring nutrients from the environment, to an organism, and back to the environment. Since energy can neither be created nor destroyed, it can only be transferred from one location to another. To maintain balance, matter is recycled between organisms and the environment.
The main elements involved in life cycles are Carbon, Hydrogen, Oxygen and Nitrogen. These are used in the synthesis of organic compounds such as carbohydrates, lipids, proteins and nucleic acids.
There are four main cycles that will be discussed in this chapter:
Also called the Hydrological cycle. It depicts the path of water through the environment, from the earth to the atmosphere and back to the earth.
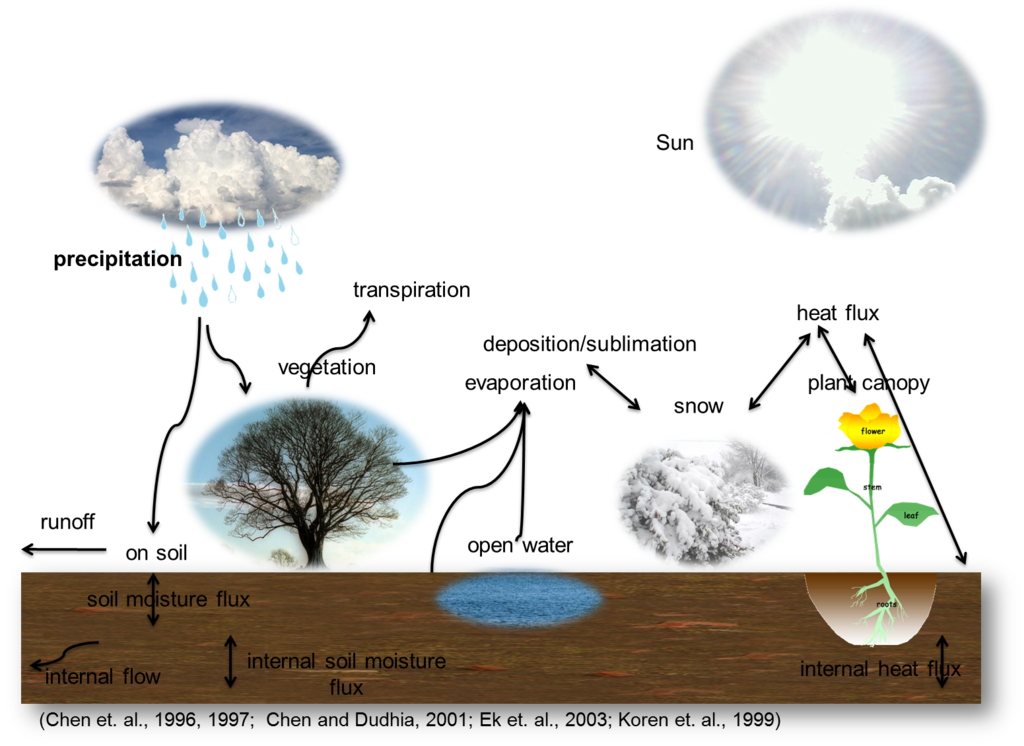
An illustration of the water cycle. (Source: Wikipedia, CC BY-SA 3.0)
Important Processes involved in the water cycle:
Because this cycle involves the flow of CO2 through the biosphere, this cycle is also referred to as Carbon-Oxygen cycle. It involves the interrelation between photosynthesis and cellular respiration.
Plants utilize carbon dioxide to produce sugars. Animals utilize these sugars, produce carbon dioxide, and other carbon compounds such as methane. Carbon dioxide is also released from other activities such as automobiles, industries, volcanoes etc.
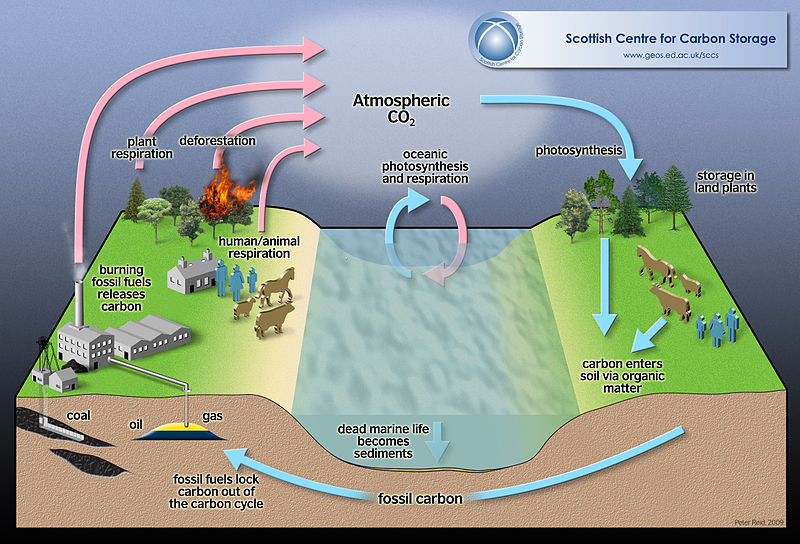
An illustration of the carbon cycle. (Source: Wikipedia, CC BY-SA 3.0, Authored by Harry C - 2009. No alterations made)
Some human activities result in production of higher carbon dioxide into the atmosphere such as burning fossil fuels, using forests for energy/fuel sources etc. These result to a phenomenon called Greenhouse effect. The Greenhouse Effect is the process by which radiation from a planet's atmosphere warms the planet's surface to a temperature above what it would be without this atmosphere.
The most common greenhouse gases in the atmosphere include:
As heat continues to be retained on the earth's atmosphere, the ambient temperatures increase resulting in global warming. Some of effects of global warming include melting ice caps, rise in sea levels, increased or reduced rainfall, change in natural vegetation, increased or reduced occurrences of natural disasters such as floods, impact on agriculture especially in cases of floods or droughts.
Ozone (O3) acts as a blanket around the atmosphere and shields/protects the earth from UV radiations from the sun. 99% of UV radiations from the sun are screened by the ozone layer and never reach the earth surface. However, the use of CFCs and other greenhouse gases has resulted in destruction of the ozone layer resulting in increased UV radiations reaching the earth surface. This may be associated with increased skin cancer.
The air in the atmosphere contains about 79% Nitrogen. Nitrogen is crucial in animal and plant tissue, especially the protein and DNA component. It is also prevalent in fertilizers used for agriculture.
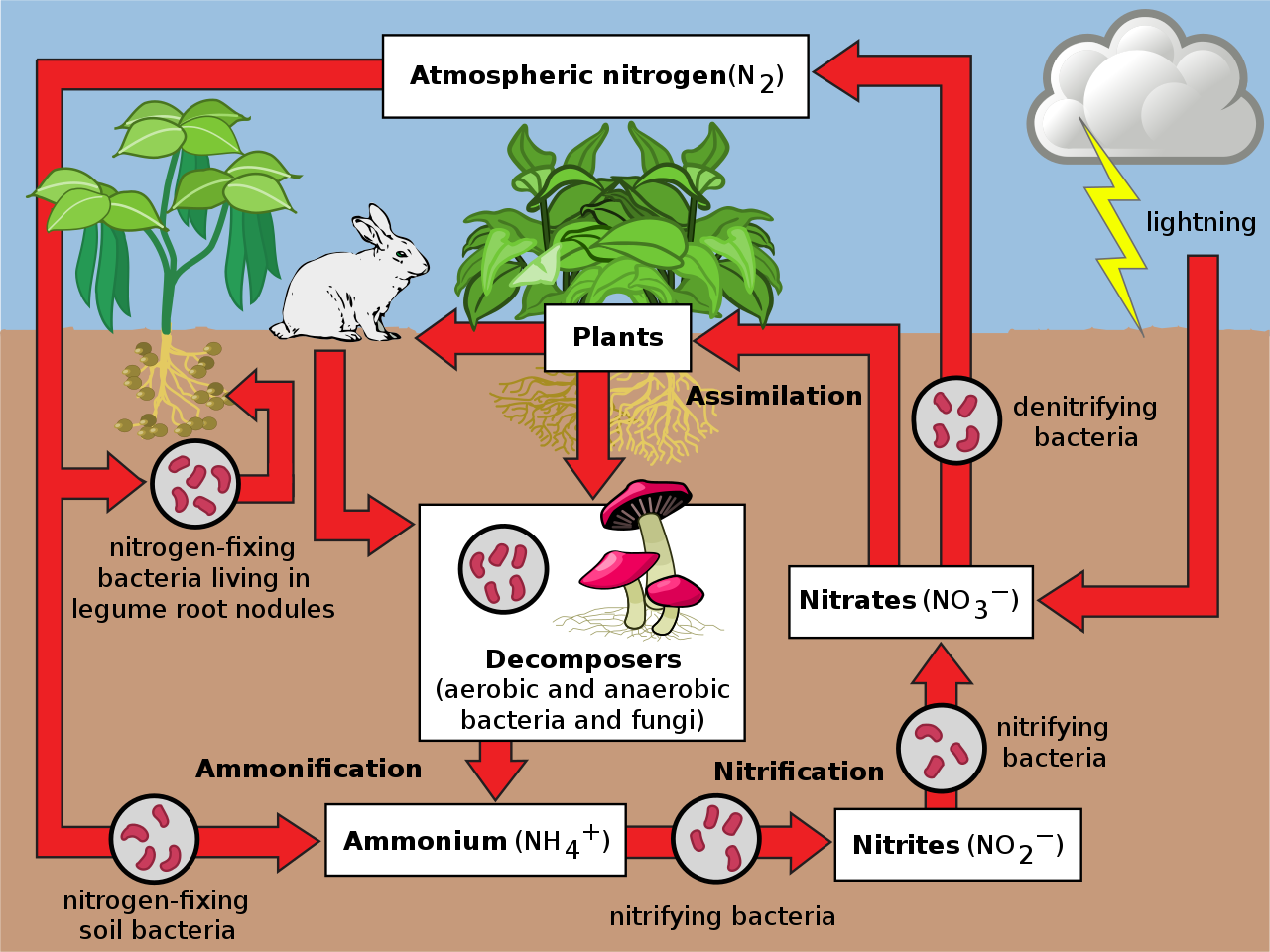
An illustration of the Nitrogen cycle. (Source: Wikipedia, CC BY-SA 3.0, No alterations made)
Nitrogen is usually utilized in life in the form of nitrates. There are 2 main ways atmospheric nitrogen can be converted into useful forms - Lightning and Legumes. Lightning can create chemical reactions between nitrogen and oxygen to form nitrates, which fall onto the ground with precipitation and be absorbed by plant roots. Plants will change these into proteins. Later, animals feed on the plants and reorganize the amino acids into the proteins for their biochemical reactions. On the other hand, legumes (bean family) have nitrogen-fixing nodules on their roots. These nodules contain symbiotic bacteria (Nitrogen fixing bacteria) that can convert atmospheric nitrogen into nitrates.
Organisms produce nitrogen as waste through urine (urea, uric acid or ammonia), feces, or after death through decay. Ammonia decays into nitrites, which further degrade into Nitrates. The nitrates can then re-enter the nitrogen cycle.
Denitrifying bacteria are a type of bacteria that are able to convert nitrates back into free nitrogen gas; which enters the atmosphere and may be through the processes already described.
In living organisms, phosphorus is found in higher concentration in bones and teeth (as calcium phosphates), DNA (phosphate group) and ATP (as tri-phosphate). Unlike the other biochemical cycles; the Phosphorus cycle does not involve movement of phosphorus in the atmosphere. The cycle can be split into an abiotic/geological component, and a biotic/living component. The geological component is dominated by phosphorus found in rocks. Being soluble in water, it dissolves and gets washed away through erosion into rivers and streams and finally to the ocean. In the ocean, phosphorus forms part of the sediments. Accumulation of phosphorus in the water bodies results in increased in plant growth (Eutrophication) and the phosphorus is transferred to the aquatic plants. Animals then eat these plants and the phosphorus is transferred to animals. Animals will revert the phosphorus back to the land when they die, as part of their decomposition.
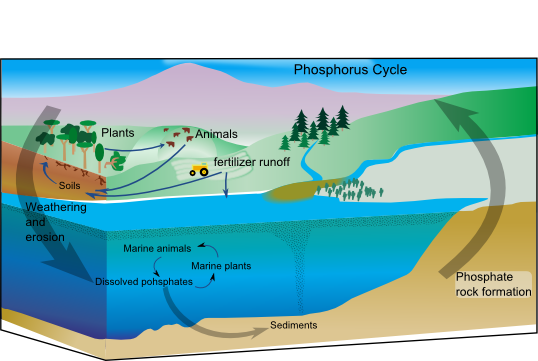
An illustration of the Phosphorus cycle. (Source: Wikipedia, CC BY-SA 3.0, No alterations made)
You can access Tensai High School Biology revision questions HERE