Macromolecules


Macromolecules are large biological molecules that perform diverse functions in the body such as energy metabolism (carbohydrates and fats), structural functions (proteins), genetics and hereditary functions (DNA and RNA), enzymes (Proteins) etc.
Macromolecules are involved in metabolism, a term that is used to describe all chemical reactions involved in maintaining the living state of the cells and the organism.
Metabolism can be divided into two categories: Catabolism - the breakdown of larger molecules (polymers) into smaller units (monomers) to produce energy, Anabolism - the synthesis of larger compounds (macromolecules) from smaller subunits.
Polymers are synthesized (polymerization) through condensation 'dehydration' reactions, by adding a monomer to a growing polymer. This process requires energy and releases water as one of the products.
Macronutrients are large, complex, organic molecules that make up, and supply energy to, cells. They are required by the body in relatively larger amounts. These include Carbohydrates, Lipids, Proteins and Nucleic acids. Micronutrients are smaller and simpler molecules and required by the body in relatively smaller amounts. These include Vitamins and Minerals.
Subsequent sections will focus on each of the macromolecules in deeper details.
Carbohydrates (hydrates of carbon) are also called 'sugars', though this shouldn't necessarily be confused with sugar (sucrose) even though, sucrose is also a carbohydrate, there are carbohydrates, such as cellulose, that aren't sugary at all.
Most carbohydrates have the chemical formula Cm(H2O)n where m and n can be different, and m should be greater than 3. It should be noted that there are several carbohydrates that do not follow this formula, but it's definitely a good guide.
The main function of carbohydrates is in energy metabolism. However, some carbohydrates play a structural function in plasma membranes and cytoplasm.
Carbohydrates can be categorized into Monosaccharides, Disaccharides and Polysaccharides.
Single sugar with the formula C6H12O6. There are three common isomers for example Glucose - blood sugar, Fructose - found in fruit, honey, twice as sweet as glucose, and Galactose - found in milk sugar, rarely found alone.
Monosaccharides are named according to the number of carbons, for example the 3 carbon sugar is called Glyceraldehyde, the 5 carbon sugar is called Ribose, while fructose, galactose and glucose are all 6 carbon sugars differing in the composition of their side chains.
Galactose and Glucose can be referred to as Isomers because they have the same chemical formula but only differ in their structural formula.
Formed by joining two monosaccharides in a dehydration reaction. For example, the reaction of Glucose and Fructose results in Sucrose, and water as a byproduct.
There are three common Isomers of disaccharides:
Sucrose = Glucose + Fructose. Example sugar cane, table sugar.
Maltose = Glucose + Glucose. Found in seeds of germinating plants.
Lactose = Glucose + Galactose. Found as the predominant sugar in milk.
Lactose intolerance: During digestion, disaccharides must be broken down to monosaccharides, then absorbed into the blood. Lactose is broken down to glucose and galactose by the enzyme lactase. People with lactose intolerance lack sufficient amounts of the enzyme. and therefore can not break down the lactose. Usually results in flatulence and stomach pain.
Purchase a pdf copy of Unit 2 (Macromolecules) for offline use.
C$0.99
Also called Complex sugars / Complex carbohydrates. They are not only polysaccharides (many monomers) but also have complex structural formulas and functions.
Some polysaccharides have an energy storage function in plants (Starch) and animals (Glycogen). Other polysaccharides have a structural function such as Cellulose (in plants) and Chitin (in animals).
Cellulose is a polymer of glucose subunits. Hydrogen bond cross-bridges make it too stable for humans to digest and requires the enzyme Cellulase found in microbes to digest it. Cellulose makes up a large component of plant cell walls (50% of organic carbon in biosphere is cellulose). Plant tissue makes up large component of the nutrition of ruminants. This explains the presence of microbes in the rumen of ruminants to enable them to digest the cellulose in their diet. in humans, the undigestible cellulose acts as fiber, which is necessary to create the 'bowel movement' that aids in the movement if digesta into fecal material. Fiber is also considered important as it makes someone feel fuller for a longer time, and could be used for weight management.
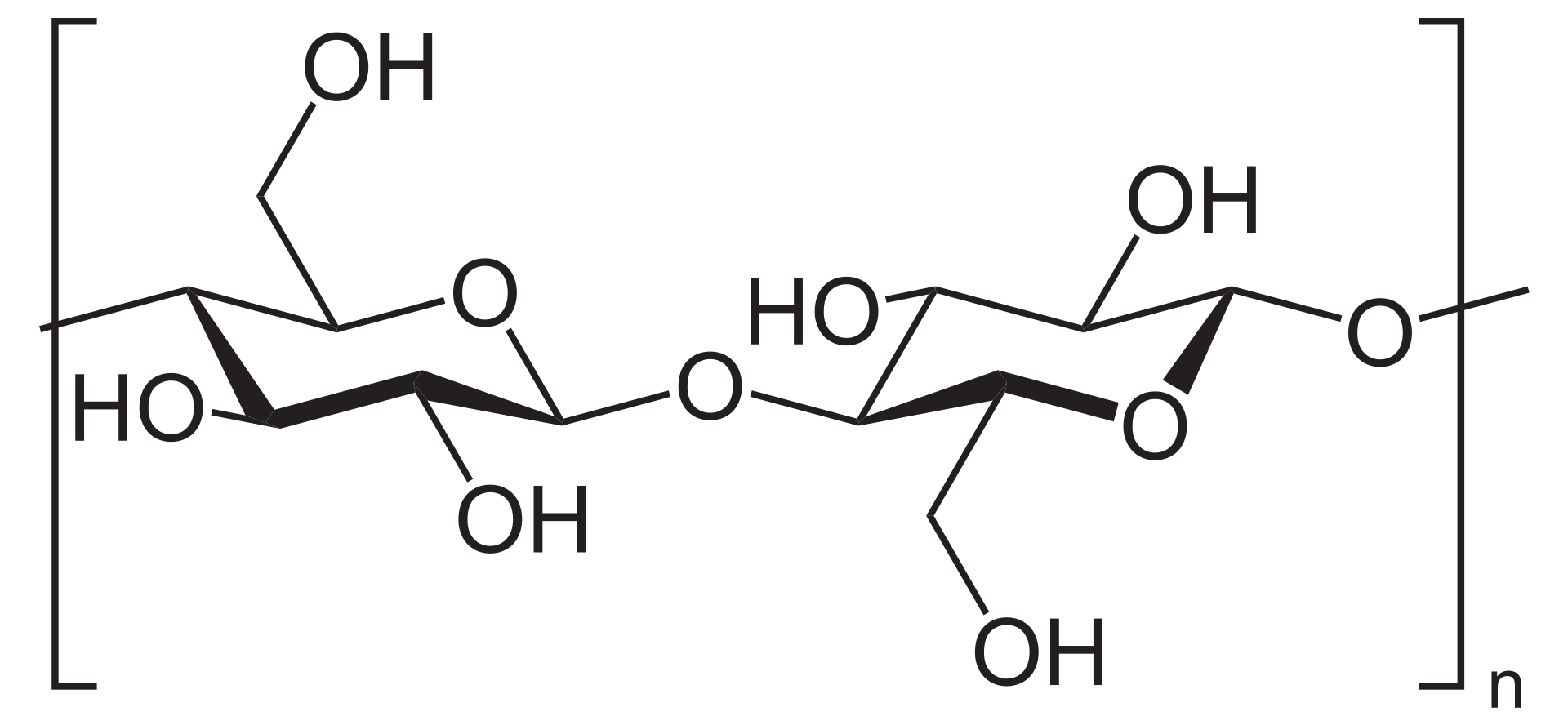
The structural formula of Cellulose seen as a polymer of Glucose. (Source: Wikipedia-CC BY-SA 3.0)
Starch also called Amylose, is also a polymer of Glucose used as a storage molecule in plants. Starch is dominantly found in tubers (such as potatoes) and grains (such as rice, corn).
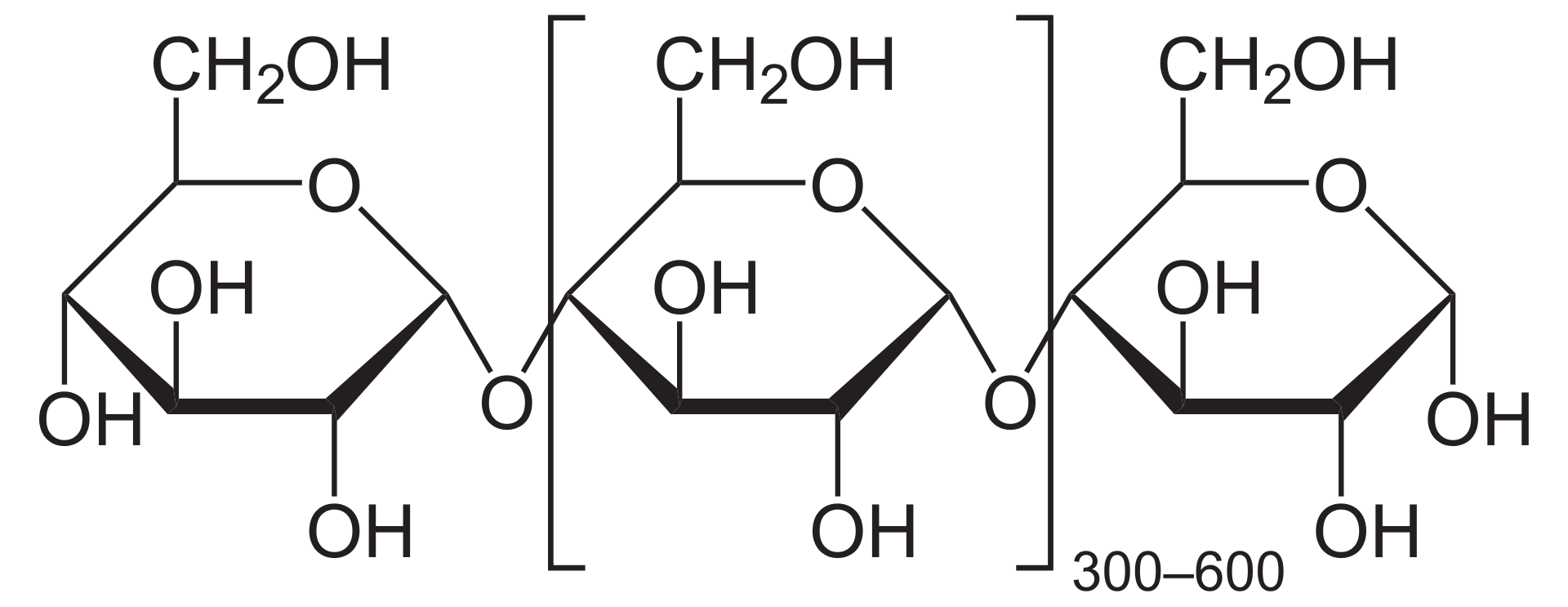
The structural formula of Starch, also seen as a polymer of Glucose. (Source: Wikipedia-CC BY-SA 3.0)
Glycogen is the energy storage molecule in animals. When blood glucose levels are high (for example after a meal), the hormone insulin causes the liver to convert glucose into glycogen, which results in lowering the blood glucose concentration. When blood glucose is low (such as during hunger or exercise), the hormone glucagon causes the liver to convert glycogen back to glucose raising the blood glucose levels.
Proteins make up about 17% of body weight. They are the second most abundant biomolecule after water. Proteins are produced from genes, so because of the diverse nature of the genetic code, proteins tend to be species-specific and individual-specific.
Cell Structure: Proteins make up major components of muscles, skin, neurons etc. They are also necessary for maintaining the cell structure through proteins such as Microtubules.
Chemical messenger: Some hormones (such as the Growth hormone) are synthesized from proteins and act as chemical messengers in various endocrine functions.
Transport: Among other examples, Hemoglobin protein is the red component in red blood cells necessary for the transport of Oxygen in blood.
Movement: Some proteins are able to contract, and thereby creating movement. The contraction of spindle fibers or the contraction of flagella make for good examples.
Cellular reactions: Enzymes are proteins that are necessary for catalyzing chemical reactions in the body.
Defense / Immunity: Think antibodies.
Proteins are classified as either Fibrous or Globular. Fibrous proteins include collagen and muscle fibers. Globular proteins include enzymes, antibodies and hormones.
Proteins (also called Polypeptides) are made of monomers called Amino Acids. Adjacent amino acids are bonded together by covalent bonds called Peptide bonds. The amino acids are arranged in a specific order that is determined by the genetic sequence present in the gene that encodes the specific protein.
There are 20 different amino acids, which can be classified as either Essential or Non-essential. Essential amino acids are those that the body cannot synthesize and must be provided in the diet. There are eight amino acids that are essential in humans.
An amino acid has a specific chemical structure, with an Amino group to one side, Carboxyl group on the other side, a Hydrogen and an R (variable) group. It is the R group that result in side chains that are different for different amino acid.
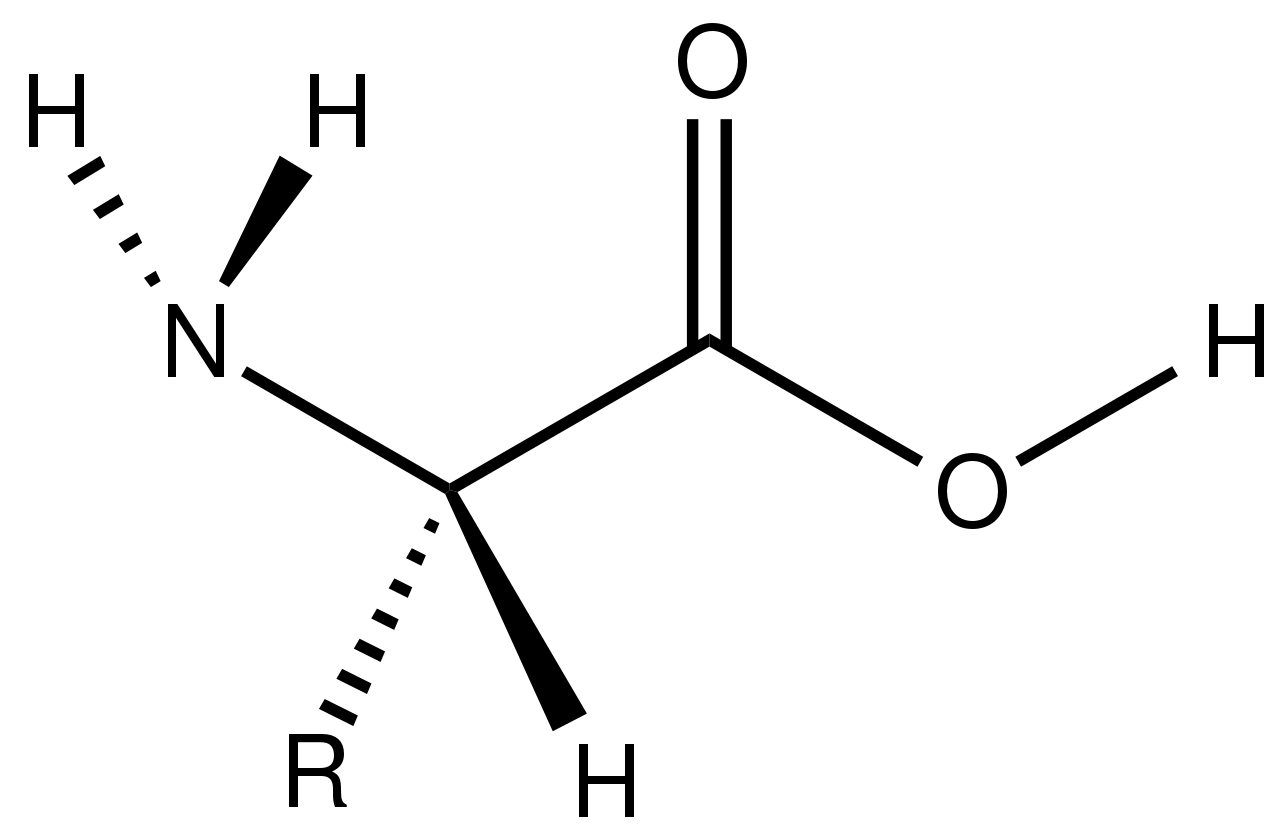
The general structure of an amino acid. (Source: Wikipedia-CC BY-SA 3.0)
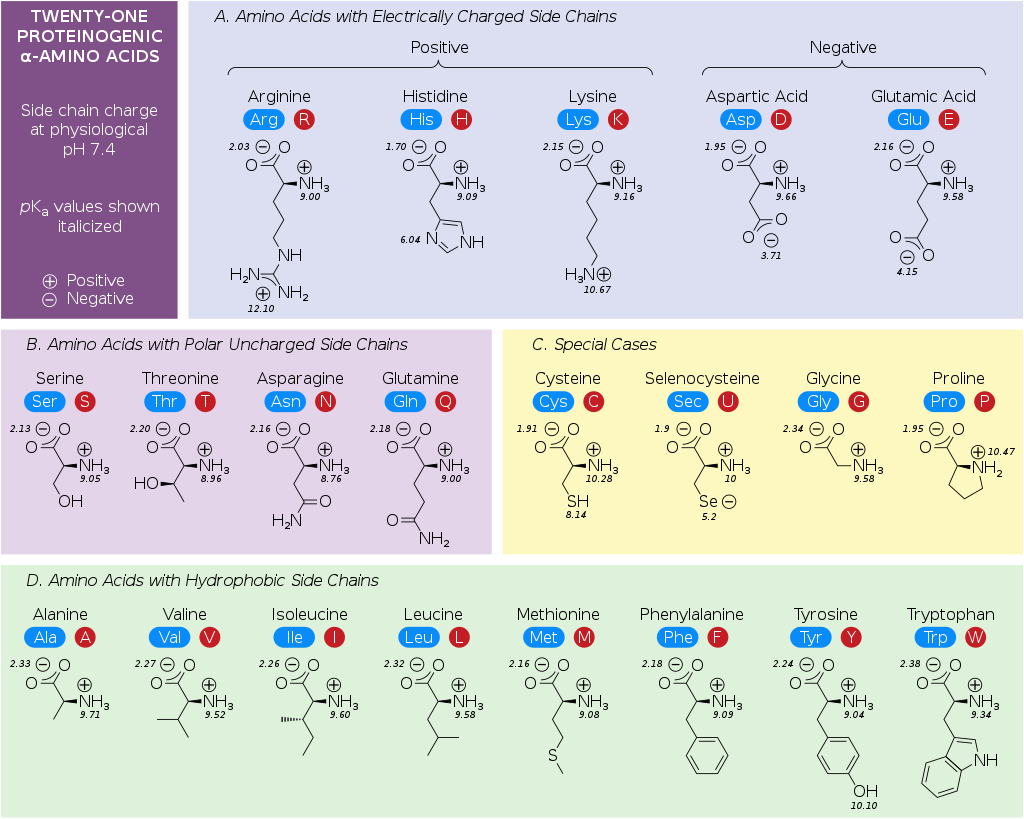
The chemical structure of all amino acids. (Source: Wikipedia-CC BY-SA 3.0)
Denaturation: occurs when the structure of the protein is altered by physical or chemical factors such as heat, radiation or pH changes. The proteinís physical and chemical properties are changed.
Coagulation: is the permanent or irreversible change in the structure of a protein, such as happens when an egg is boiled.
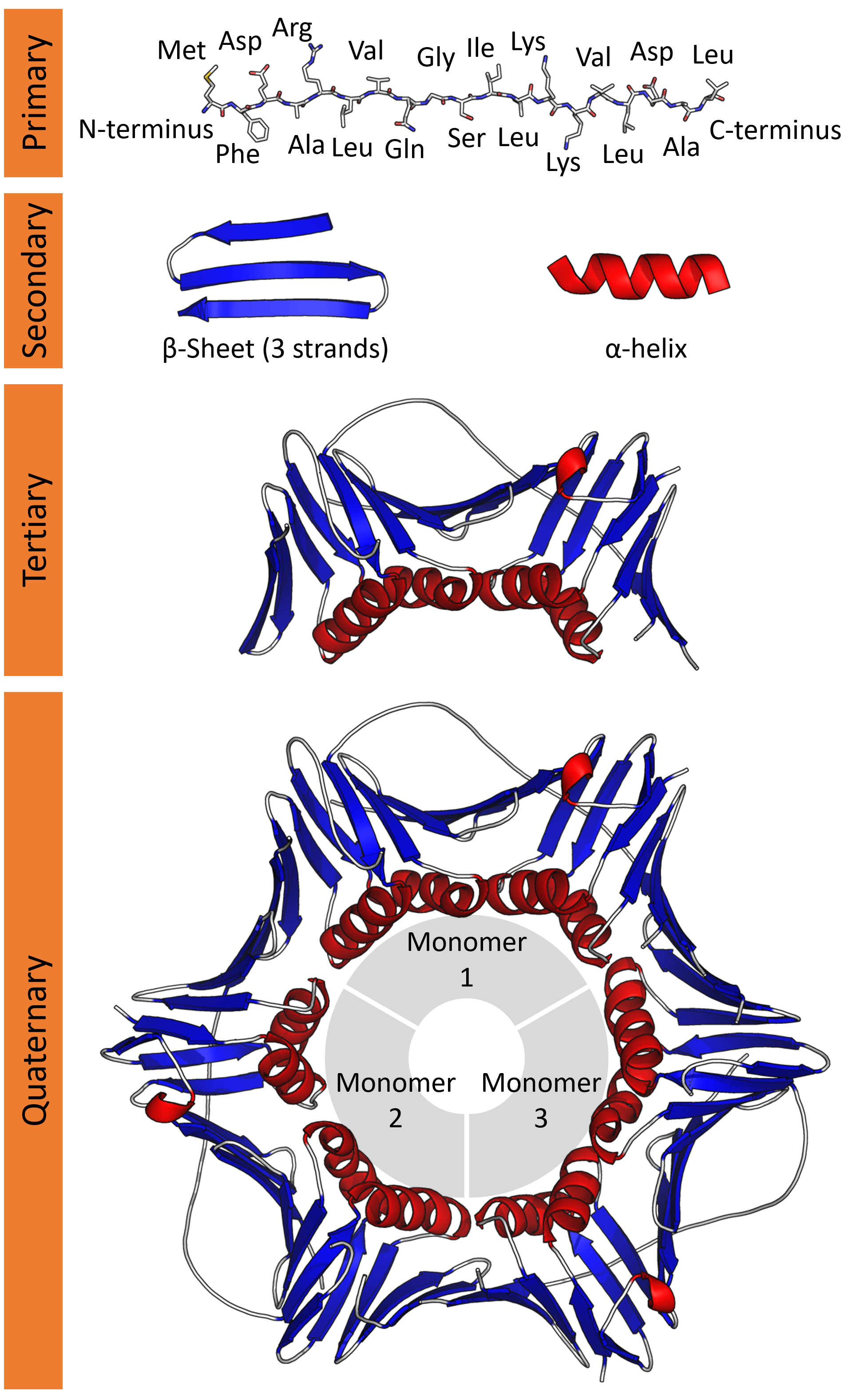
The structure of proteins can be broken down into 4 levels, as follows:
Nucleic acids are polymers of nucleotide monomers. A nucleotide is unit with three components; a nitrogenous base, a phosphate group and sugar. The combination of the nitrogenous base and the sugar (without the phosphate group) is referred to as a nucleoside.
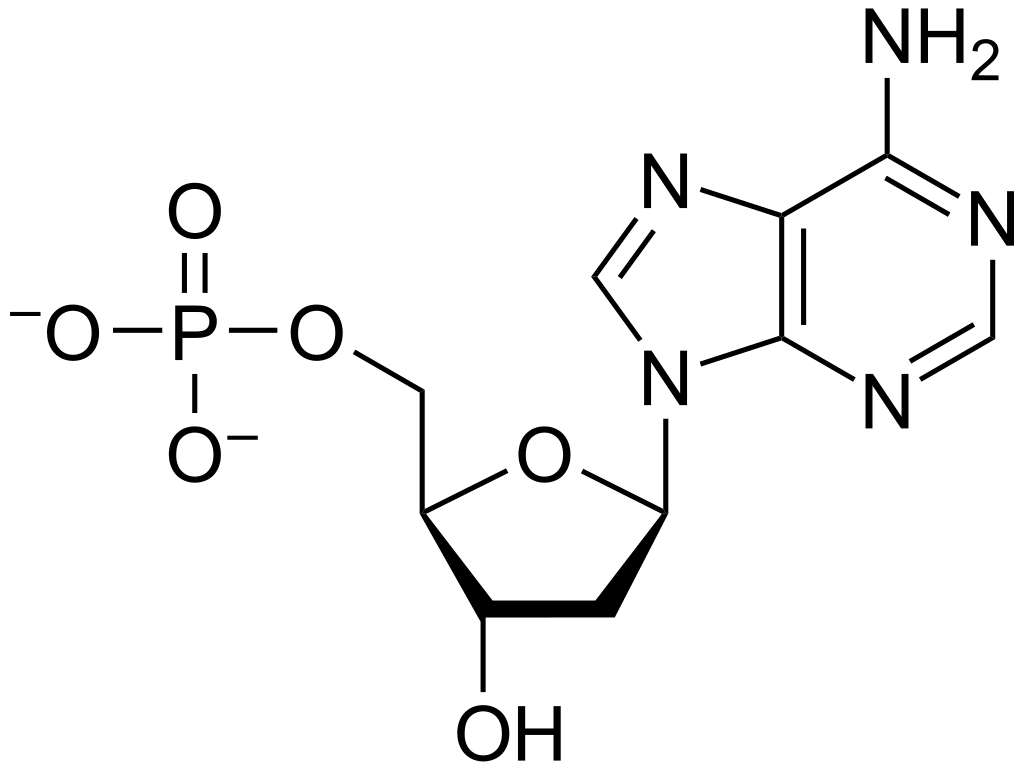
The chemical structure of a nucleotide with the 5-carbon sugar in the middle, a phosphate group on one side and a nitrogenous base on the opposite side. (Source: Wikipedia-CC BY-SA 3.0)
There are two types of Nucleic acids: Deoxyribonucleic acid (DNA) and Ribonucleic Acid (RNA). The following are the major differences between these two types:
Deoxyribonucleic Acid (DNA)
Ribonucleic Acid (RNA)
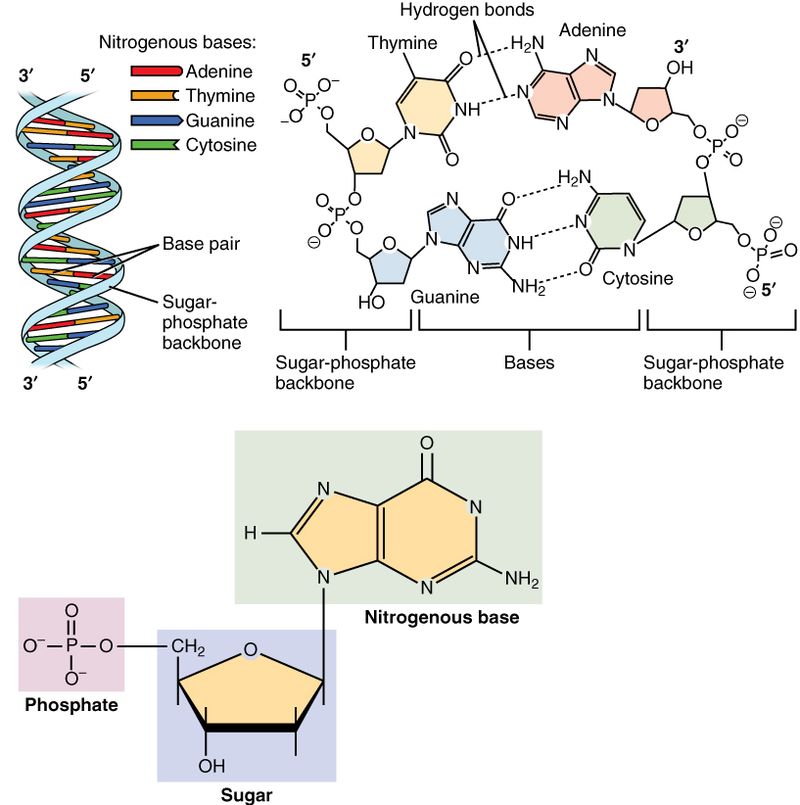
DNA is a polymer of nucleotide monomers (Source: Wikipedia-CC BY-SA 3.0)
Lipids (fats, oils, waxes) consist of two components a glycerol backbone, which is a 3-carbon molecule, and fatty acids connected through the carbons in the Glycerol molecule. Lipids can also be referred to as Triglycerides or Triacyglycerol. As expected, lipids are insoluble in water
Simple Lipids: Triacylglycerol. They are mostly utilized by the body for energy metabolism.
Complex Lipids: Mainly structural functions such as plasma membranes. Include Phospholipids and Glycolipids.
Derived Lipids: These include cholesterol, Eicosanoids and Carotenoids.
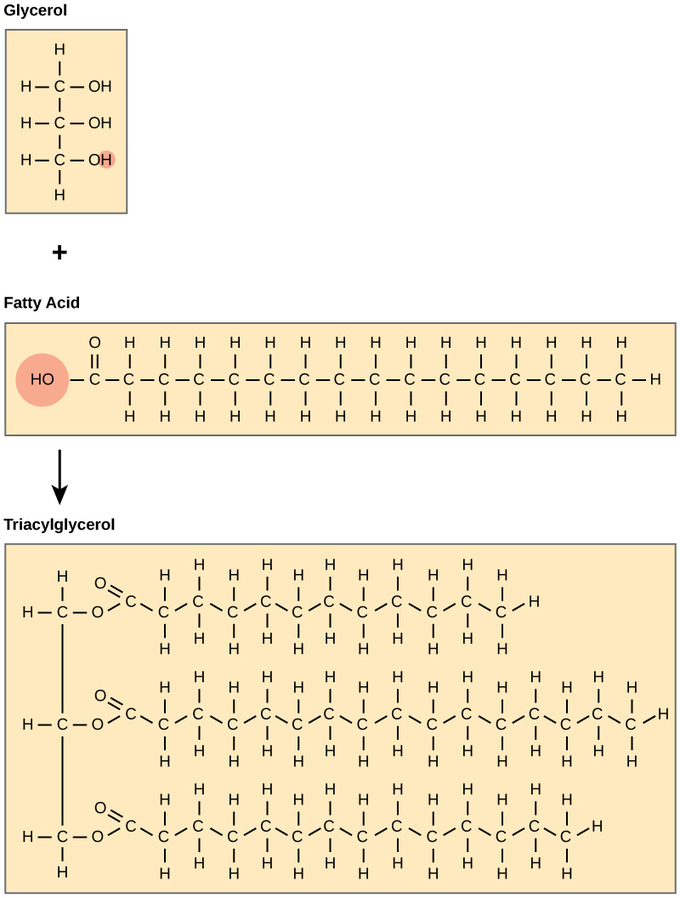
The chemical structure of a glycerol molecule, a fatty acid and a triacylglycerol. (Source: Wikipedia-CC BY-SA 3.0)
For example Butter. They are called saturated because they only contain single bonds in the fatty acids. Saturated fats are usually from animals, they are solid or semi solid at room temperature and are very stable, making it hard to break down.
These are oils (such as canola oil, corn oil, sunflower oil etc.) because they are liquid at room temperature. They are obtained from plants. They are described as unsaturated because they contain at least one double bond in the fatty acid molecule. Polyunsaturated fatty acids (PUFA) have several double bonds between carbon atoms making them more reactive than fats therefore more easily broken down.
In Phospholipids, one of the fatty acid chains in a triacylglycerol molecule is replaced by a phosphate group resulting in only two fatty acid chains. Because of the presence of the phosphate group, one end of the phospholipid, the phosphate head end, is soluble in water (hydrophilic) while the other end, the fatty acid tail, is insoluble in water (hydrophobic). When phospholipids make up cell membranes, because of their interaction with water, only the phosphate heads can be oriented towards the water rich side of the cell, resulting in a bilayer, where the tails are in the center and the heads are on the outside.
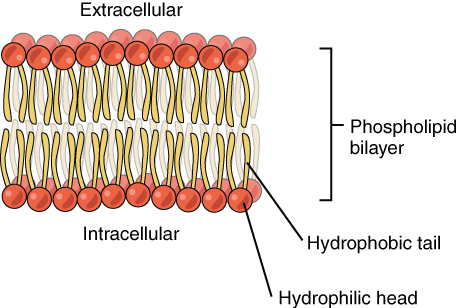
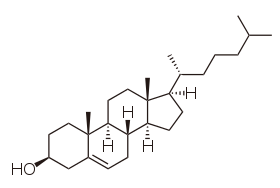
The chemical structure of a phospholipid plasma membrane bilayer and Cholesterol. (Source: Wikipedia-CC BY-SA 3.0)
Steroids are made from cholesterol. They consist of four fused carbon rings and their function is mostly hormone synthesis. They are also present in bile and in plasma membrane. On the plasma membrane, cholesterol is necessary to influence the viscosity of the membrane.
Waxes are fatty acids attached to carbon rings. They are insoluble in water making them good for waterproofing or protecting materials from water damage. In plants, waxes are present in the cuticle where they prevent water loss. Waxes are present in earwax where they prevent foreign materials from entering the ear.
Benedicts Reaction is used to tests for the presence of simple sugars.
Negative test: After heating the Benedict solution remains blue.
Positive test: After heating the blue Benedict solution turns yellow to orange.
Benedict reagent can be used to test for presence of glucose in urine as an indicator of Diabetes.
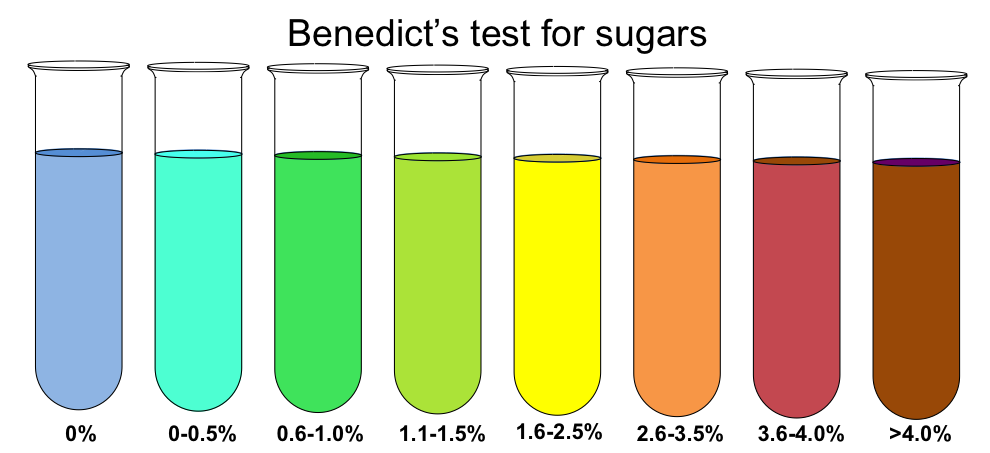
Benedict reaction in varying levels of glucose content in the sample. (Source: Wikipedia-CC BY-SA 3.0)
Negative Test: The iodine solution remains amber when no starch is present.
Positive Test: The iodine solution turns blue black when starch is present.
Ordinary Biuret solution is blue.
Negative Test: When added to a substance not containing protein, the solution remains blue.
Positive Test: When added to a substance containing protein, the substance turns purple.

The appearance of a positive Biuret test. (Source: Wikipedia-CC BY-SA 3.0)
The translucence test involves rubbing samples on a piece of unglazed paper and observing a translucent spot as the confrimation of the presense of fats.
The Sudan IV test involves adding Sudan IV dye to the sample. If fat is present in the sample tested, a red or pink color will result.
Please note that the Sudan group of dyes (Sudan I, Sudan III, Sudan IV) have been found to be carcinogenic and should be handled with care.
You can access Tensai High School Biology revision questions HERE